Insights Archive
Filter by:
April 25, 2025
Drug Spotlight: Yesintek and Selarsdi
YESINTEK (ustekinumab-kfce)Manufacturer: BioconLaunch Date: 2/24/2025 SELARSDI (ustekinumab-aekn)Manufacturer: Alvotech/TevaLaunch Date: 1/2025 YESINTEK & SELARSDI are biosimilars of reference product STELARA (Ustekinumab),…

November 4, 2024
Neffy® (brand name: epinephrine nasal spray)
Neffy® (epinephrine nasal spray) was approved on August 9, 2024, for the emergency treatment of allergic reactions (type I), including those that are life-threatening (anaphylaxis), in adult and pediatric patients weighing at least 66 pounds.
July 15, 2024
Drug Spotlight: liraglutide (brand name Victoza)
On June 24, 2024, drug manufacturer Teva Pharmaceuticals announced the launch of an authorized generic formulation of Novo Nordisk’s Victoza (liraglutide). This marks the first generic glucagon-like peptide-1 (GLP-1) agonist available in the U.S. market.
April 3, 2024
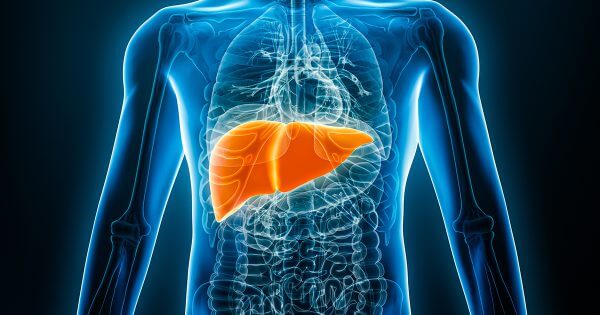
Rezdiffra (resmetirom)
On March 14, 2024, drug manufacturer Madrigal Pharmaceuticals announced the approval of Rezdiffra™, a new, first in class oral therapy for nonalcoholic steatohepatitis (NASH). This disease, which impacts an estimated 6-8 million people in the U.S., is underdiagnosed and if left untreated, it can cause serious liver complications. Read more about this approval and its implications.

January 11, 2024
New Drug Spotlight: Zepbound
On November 8, 2023, Eli Lilly announced the approval of the newest weight loss drug Zepbound, a much-anticipated formulation of tirzepatide in the high-profile GLP-1 agonist medications class. Used along with reduced calorie diet and exercise, studies have shown Zepbound can lead to significant weight loss.

October 2, 2023
New Drug Spotlight: Arexvy, Abrysvo, and Beyfortus
In 2023, the U.S. Food & Drug Administration (FDA) approved three new vaccines for the prevention of Respiratory Syncytial Virus (RSV). RSV is a highly contagious, common respiratory virus that typically causes mild cold-like symptoms. However, it can cause serious illness in older adults and infants.

May 30, 2023
New Drug Spotlight: Amjevita
The launch of Amjevita, the first FDA-approved biosimilar for Humira, is big news for pharmacy benefit plan sponsors.
